Location: Home >> Detail
TOTAL VIEWS
J Psychiatry Brain Sci. 2025;10(3):e250003. https://doi.org/10.20900/jpbs.20250003
Department of Psychiatry, Washington University School of Medicine, St. Louis MO 63110, United States
* Correspondence: Pamela N Romero Villela.
Background: Chronic pain (CP) and substance use disorders (SUDs) frequently co-occur. This brief review highlights environmental, neurobiological, and genetic sources of comorbidity of CP and SUDs, focused on alcohol, nicotine, cannabis, and opioids.
Methods: A literature search on CP and SUDs was performed using Google Scholar and PubMed. Relevant literature was summarized in a narrative review.
Results: Recent genomic studies reveal that SUDs and CP share a significant portion of genetic variance, and causal inference methods suggest that CP and SUDs have bidirectional effects on one another. CP and SUDs share multiple neurobiological pathways such as the reward and stress systems, with studies implicating important regions such as the insular and anterior cingulate cortex, the ventral tegmental area, and the nucleus accumbens. Environmental risk factors for CP and SUDs include socioeconomic background, education, and broader environmental factors such as neighborhood resources, air quality and greenspace. Social support is also a protective factor against CP and SUD diagnoses and crucial for their successful treatment and remission.
Conclusions: Promising new areas of research underlying CP and SUD comorbidity include female-specific CP conditions and substance use patterns, the role of the immune system in both SUDs and CP, and the rise of large biobanks that will further precision medicine by allowing researchers to jointly model genetic, neurobiological, and socio-environmental factors underlying their co-occurrence. In summary, CP and SUDs are debilitating conditions with far-ranging consequences for both individuals and communities; investigating their shared etiology will result in better treatments for both.
CP, chronic pain; US, United States; SUDs, Substance use disorders; OUD, opioid use disorder; AUD, alcohol use disorder; CUD, cannabis use disorder; TUD, tobacco use disorder; MCP, multisite chronic pain; GWAS, genome-wide association study; addiction-rf, addiction risk factor; MR, mendelian randomization; PTU, problematic tobacco use; HPA axis, hypothalamus, pituitary, and adrenal glands as part of the brain’s stress system UK, United Kingdom
Chronic pain (CP) is broadly defined as pain lasting three months or more [1]. CP is common, affecting 33% of the worldwide [2] and 24.3% of the United States (US) population [3]. It is also highly heterogeneous, with a wide range of potential causes, from injury to surgery to neurological disease. Severe CP can impair day to day activities [4], lead to depression [5], and result in long-term physical disabilities [6]. CP also burdens national healthcare and economic systems, costing between $560–$635 billion annually in the US alone [2]. Substance Use Disorders (SUDs) are generally defined as continued, compulsive substance use, even in the face of social and physical consequences, to alleviate uncomfortable feelings when not using the substance [7]. Like CP, SUDs are also relatively common (depending on the substance) and present a major public health burden, costing the US over $13 billion in 2017 alone [8]. According to the Centers for Disease Control, around 14.5% of people in the United States over the age of 12 have a SUD [9]. Individuals suffering from SUDs often use multiple substances (polysubstance use [10]), and SUDs are frequently comorbid with other mental [11] and physical health conditions, including depression [12], cardiovascular disease [13], and chronic pain [14].
CP and SUDs co-occur frequently; studies report that around 40% of individuals suffering CP also met criteria for SUD [15]. Co-occurring pain further complicates SUD recovery; for example, a study [16] found that 20.6% (7.6 million people) of adults in the US with a past year SUD diagnosis reported having pain interfering with their SUD recovery. Therefore, understanding the mechanisms by which CP and SUDs co-occur is important to develop effective treatments for patients suffering from both diagnoses and ensure patients continue being successful in their SUD remission efforts.
This narrative review highlights the genetic, neurobiological, and environmental risk and protective factors that are shared between CP and SUDs and may explain their co-occurrence (for overview, see Figure 1). Throughout, the focus is on alcohol, nicotine, cannabis, and opioids, as these are the most used substances in the United States and the most well-studied in terms of their genetic etiology and connections with CP. First, evidence of genetic overlap between CP and SUDs will be reviewed. Next, this review will discuss shared neurobiological mechanisms, followed by environmental sources of CP and SUD comorbidity. Finally, the review will conclude with a summary of key findings.
Opioid use disorder (OUD) is the most well-known SUD in the context of comorbidity with CP [17]. Opioids are often prescribed to manage pain, and an estimated 3%–4% of adults in the US have had a long-term opioid prescription [17]; approximately 35%–12% of adults using opioids to treat chronic pain will develop opioid use disorder or abuse [14]. Males and younger individuals are at greater risk of developing OUD after being prescribed opioids to treat CP, and those with personal or family history of SUDs are also at increased risk [14]. Other factors that may increase risk include negative affect (i.e., anxiety or depression) and self-reported opioid craving [14]. It is important to note that while opioid prescriptions have decreased in the US in recent years, the rate at which opioid overdose deaths have decreased varies greatly depending on the kind of opioid used. For example, while deaths due to prescription opioids and heroin have generally remained steady or declined since around 2017, overdose deaths due to fentanyl and other synthetic opioids had been increasing sharply until 2023 (the “third wave” of the opioid crisis) [18,19]. For the first time since 2018, total drug overdose deaths decreased from 2022 to 2023, including a decrease in deaths due to synthetic opioids such as fentanyl, which showed a decrease of 2.2% [20]. During that same year, the drop in deaths due to heroin was much sharper, around 33.3% [21]. Further research is needed to understand differential risk for addiction and overdose deaths across opioid subtypes.
While the development of OUD remains a concern in patients prescribed long-term opioid therapy, this concern must be balanced with the need to provide appropriate and effective pain treatment to those experiencing CP. Despite the attention that the opioid crisis has gathered, medical schools continue to lack robust pain management training; what is more, even fewer teach about addiction [22]. Lastly, racial disparities in opioid treatment of CP must also be addressed: despite studies suggesting that Black [23] and Hispanic [24] individuals may experience higher rates of CP and pain intensity, studies suggest that Black individuals are often under prescribed opioids for CP management by over 36% compared to their White counterparts [25]. Gaining a better understanding of risk factors that increase one’s liability to develop OUD after long-term opioid therapy and developing alternative, effective pain management strategies are crucial for improving prevention of OUD and treatment of CP.
Alcohol is one of the most widely used substances across the world [26] and may provide some temporary pain relief thanks to its analgesic effects [27]. However, using alcohol for its analgesic properties may then lead to increased tolerance and a need for greater alcohol consumption to achieve the same effects [28]. While low and moderate alcohol use may provide immediate analgesic effects, prolonged use is associated with worse pain trajectories over time [29]. Individuals with problematic alcohol use are more likely to experience pain than those without problem use [30], and prolonged alcohol use is a leading cause of neuropathy [31]. In addition, depression may serve as a mediator between CP and alcohol use disorder (AUD), as individuals with CP are more at risk for depression [32] and may drink alcohol to alleviate negative feelings (i.e., negative urgency, the tendency to act impulsively or engage in risky behaviors to assuage negative emotional states [33]).
Cannabis is the most widely used illicit substance across the world, with rates of use around 2.5% [34]. In the US in particular, increased legalization of cannabis has led to an uptick in recreational and medicinal use [35]. With increased legalization of both medicinal and recreational cannabis in the US, cannabis has become an alternative pain therapeutic. One study found that 3 in 10 individuals experiencing CP reported using cannabis to treat their pain [36], despite mixed evidence of the true efficacy of cannabis for treating pain. In population-based [37] and veteran samples [38], adults with CP were significantly more likely to have cannabis use disorder (CUD) compared to their non-pain counterparts. However, a cross-sectional study of patients with CP in Germany found that although 29.9% of patients with CP also reached DSM-IV criteria for CUD, the percentage of patients with comorbid CP and CUD dropped to 2.1% after removing positive behavior items (e.g., tolerance, strong desire and withdrawal from cannabis) from the CUD criteria [39], suggesting that CUD diagnoses in patients with CP might be overestimated when using only DSM-IV criteria for CUD but underdiagnosed by physician observations alone.
While tobacco smoking has generally declined in the US, the prevalence of tobacco use in CP patients remains relatively high (e.g., an estimated 35% of individuals with chronic neck or back pain use cigarettes, which is almost 3 times greater than the national average [40]). While tobacco use may provide short-term analgesic properties, nicotine can also increase pain sensitivity; individuals with CP who use tobacco report worse symptoms and greater pain severity than those who do not ingest tobacco [41]. According to a review [42] on tobacco and pain by LaRowe and Ditre, most studies investigating the link between CP and tobacco have been focused on tobacco use rather than tobacco use disorder (TUD). However, Zvolensky et al. [43] found that lifetime CP incidence was higher among individuals with nicotine dependence than non-dependent nicotine users. This study suggests that the comorbidity between CP and SUDs such as TUD merit further investigation, over and above what has been learned by studying co-occurring pain and substance use behaviors.
Genetic studies of CP and SUDs are useful for identifying biological mechanisms and/or biomarkers underlying both conditions. Additionally, genetic studies can aid in risk stratification and treatment protocols for individuals suffering from CP and at risk for developing an SUD. Genetic variants can also be used to aid in quasi-experimental Mendelian Randomization (MR) studies [44] to estimate causal relationships between CP and SUDs (and vice versa; See Table 1 for definitions of common genetics terms, including MR, used throughout this section).
To our knowledge, there are no traditional genetic twin studies on the comorbidity of CP and SUDs. Modern molecular genetic studies of CP and SUDs have demonstrated a moderate proportion of shared genetic effects. A recent study by Koller et al. [45] investigated the genetic correlation between multi-site chronic pain (MCP, a score summing the number of body sites an individual experiences CP in, ranging from 0–7) and a variety of SUDs. Genetic correlations between MCP and SUDs ranged from rg = 0.20 for opioid use disorder to rg = 0.37 for CUD. These estimates are in line with other studies such as Deak et al. [46], which estimated the genetic correlations between OUD and CP phenotypes to range from rg = 0.22 (last month: headache) to rg = 0.63 (neck/shoulder pain for 3+ months). Similarly, Toikumo et al. [47] found that the genetic correlation between TUD and MCP was rg = 0.36. In short, recent studies suggest moderate genetic correlations between specific SUDs and CP.
One study examined the genetic liability for SUDs broadly by conducting a multivariate genome-wide association study (GWAS) of general addiction liability (addiction-rf) [48]. This study found that genetic liability for the general addiction factor was significantly associated with CP, in line with the shared neurobiological mechanisms between CP and SUDs elucidated by human and animal studies [19,49]. Interestingly, another study [50] found that after controlling for the genetic effects of tobacco use disorder, there remained a significant association between genetic liability for cannabis use disorder (CUD) and musculoskeletal pain. Taken together, these findings suggest that CP may have genetic risk that is shared across SUDs (i.e., general addiction liability) and shared genetic risk that is specific to certain SUDs.
The Koller et al. study mentioned above [45] also performed pleiotropic analyses, which revealed dozens of genetic loci that influence both MCP and SUDs. Specifically, authors identified 25 loci underlying MCP and AUD, 22 for MCP and CUD, and 4 loci for MCP and OUD. However, no pleiotropy was detected at the gene or pathway level, potentially suggesting differential genetic effects within genes and/or small effects spread across multiple genes, thereby limiting the statistical power to detect such effects at the gene level. Future analyses with greater statistical power may identify pleiotropic variants implicating shared neurobiological pathways between CP and SUDs, such as stress regulation. Koller et al. also employed MR, a causal inference method, to investigate the causal relationship between CP and SUDs. Authors found evidence for causal bidirectional relationships between MCP and AUD, CUD, and Problematic Tobacco Use (PTU). However, they noted that the results were stronger for MCP influencing CUD and PTU rather than vice versa. These results are in line with another recent study [51] that found that genetic liability for chronic back pain caused increased alcohol use and smoking quantity and vice versa. Notably, Koller et al. [45] failed to identify causal relationships between MCP and OUD; authors speculated this was due to low power. Therefore, while additional studies are needed, current research suggests significant bidirectional relationships between genetic risk for CP and SUDs.
Future DirectionsLarge-scale biobanks such as the All of Us Research Program [52] and the United Kingdom (UK) Biobank [53] have collected data from around 500,000 individuals in the US and UK. These data are rich and include electronic health records, health and behavior surveys, accelerometers, and, notably, whole genome data. Until recently, datasets with rare genetic variant information available had been scarce due to limitations in sequencing technologies and high costs; accordingly, genetic studies of rare variants underlying the co-occurrence of CP and SUDs are limited [54]. Now, these large-scale biobanks have rare variant information for hundreds of thousands of individuals, enabling rare variant studies of both diseases. Studies of rare variants might implicate novel biological pathways or elucidate the core genes involved in CP-SUD comorbidity, given that rare variants are more likely to reside within essential genes and gene pathways [55]. Genetic interaction (gene-gene and gene-environment) studies investigate how a genetic factor’s effect depends on another genetic or environmental factor. To our knowledge, no studies investigating gene-gene or gene-environment interactions underlying CP-SUD co-occurrence have been reported. (See Table 1 for definitions of common genetics terms.) Both rare variant and genetic interaction studies can improve treatment of CP-SUD comorbidity by contributing to personalized treatment strategies depending on an individual’s genetic makeup (rare variants being particularly important for individual risk prediction) and their environment. Moreover, given the diversity and varied presentation of chronic pain conditions and substance use disorders, future studies using deeply phenotyped samples might be better equipped to pinpoint the genes and biological pathways underlying the co-occurrence of certain types of CP and SUDs (e.g., musculoskeletal pain and cannabis use disorder). Lastly, studying genetic variability in individual responses to drugs or treatments, or pharmacogenetics, may be key to understanding why some people who are prescribed opioids to treat CP develop OUD, while others who are prescribed opioids never develop an addiction. For example, one GWAS found a variant near the OPRM1 gene that moderated therapeutic methadone dose in individuals of African ancestry [56]. Future studies of cases with CP who develop SUDs compared to controls with CP who do not develop SUDs may shed more light on genetic variation underlying sensitivity to substances following chronic pain. In short, these rich datasets with genotypic, phenotypic, pharmacological, and medical information will allow for modeling both biological (rare and common genetic factors, biomarkers) and environmental (social determinants of health, trauma and stress) factors (and their interactions) in predictions of risk, consistent with recent work that showed that including social determinants of health into machine learning models improved the prediction of high-impact chronic pain [57]. Given the large amount of heterogeneity in the risk factors, clinical manifestation, and symptom trajectory of both CP and SUDs, precision medicine approaches are needed to provide the most effective treatments.
The stress and reward systems are complex, interconnected brain systems responsible for regulating emotion [58,59], motivation [60,61], and behavior [62,63]. The stress system is located both within the central nervous system (i.e., the brain and spinal cord) and peripheral organs [64]. Within the brain, the structures involved in the stress system include the hypothalamus, pituitary, and adrenal glands (collectively known as the HPA axis); the stress system is additionally composed of the hippocampus, amygdala, and prefrontal cortex (see Figure 2). When individuals experience CP, their stress system is oftentimes chronically activated [65]. In addition, Vachon-Presseau et al. [65] found that pain intensity in individuals with chronic back pain was associated with reduced hippocampal volume [NO_PRINTED_FORM]. Individuals suffering from a SUD also suffer from heightened, prolonged stress [66]. Therefore, given the importance of the stress system for both CP and SUDs, it is likely also a crucial neurobiological factor underlying the CP-SUD comorbidity.
The reward system responds and processes rewarding stimuli, leading to positive reinforcement and pleasurable feelings [59,63]. The reward system is key to learning and motivation. Some of the main brain areas involved in the reward system that will be highlighted here include the ventral tegmental area (VTA), nucleus accumbens, prefrontal cortex, amygdala, and hippocampus. Extensive research has implicated the VTA and nucleus accumbens in the reward and learning processes underlying SUDs [67]. The VTA resides in the midbrain and is involved in learning, memory, stress regulation, emotional processing, alongside sleep and alertness [68]. Dopaminergic activation of the VTA has been found to provide pain relief to mice experiencing models of neuropathic chronic pain [19]. Similarly, in a rat model for chronic inflammatory pain, Ezzatpanah et al. [69] found that blocking the orexin receptors in the VTA led to a dose-dependent reduction in formalin-induced biphasic pain. Interestingly, CP may increase inhibitory neurons that project unto the VTA dopaminergic neurons, thereby making these neurons less excitable and leading to other conditions such as anhedonia [70], which is a psychological condition characterized by a significant decrease in interest in things or activities that were once rewarding [71]. Anhedonia is typical among individuals suffering from substance abuse and significantly impacts the chances of relapse among individuals in remission [72].
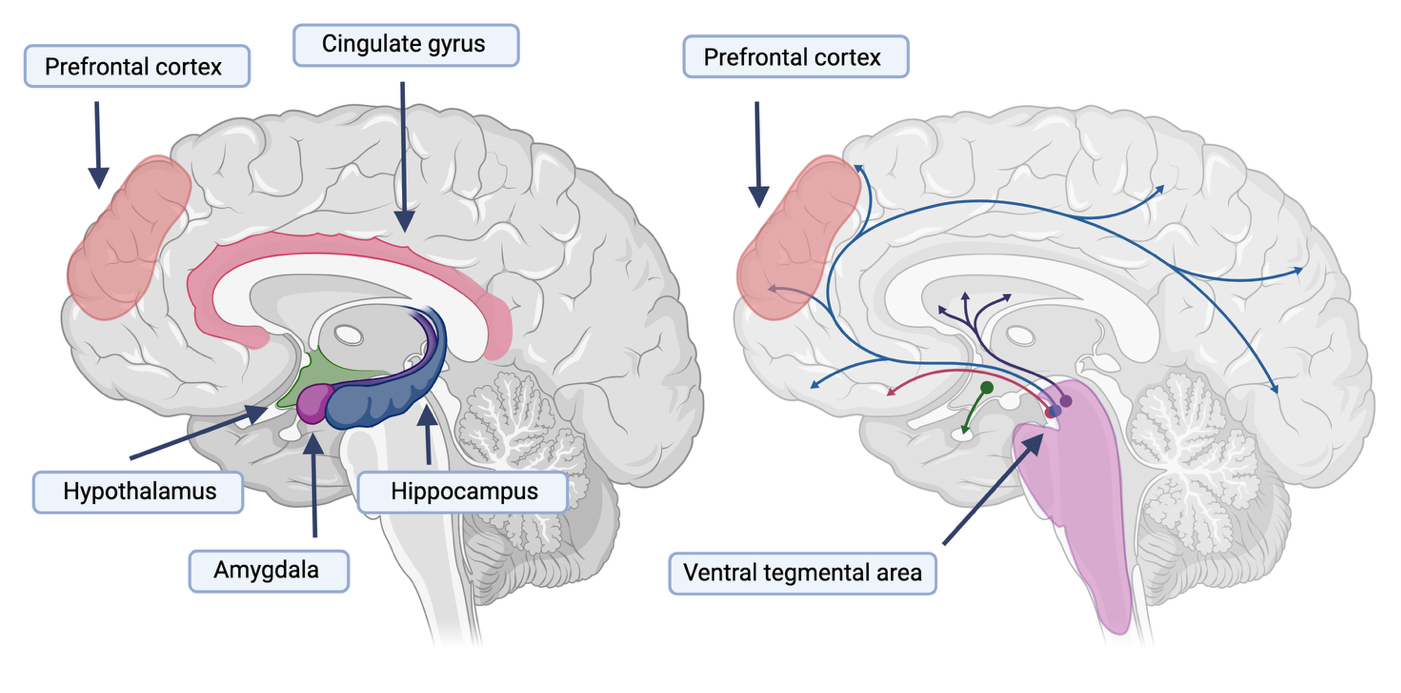 Figure 2. The stress and reward systems associated with both chronic pain and substance use disorders. On the left panel, we show some of the major brain regions associated with responding to stress, including the hypothalamus, amygdala, hippocampus, and prefrontal cortex. On the right, we show dopaminergic projections in the brain associated with the brain’s reward system and highlight the projections starting in the ventral tegmental area and going to the prefrontal cortex. Both systems have been implicated in SUDs and CP.
Figure 2. The stress and reward systems associated with both chronic pain and substance use disorders. On the left panel, we show some of the major brain regions associated with responding to stress, including the hypothalamus, amygdala, hippocampus, and prefrontal cortex. On the right, we show dopaminergic projections in the brain associated with the brain’s reward system and highlight the projections starting in the ventral tegmental area and going to the prefrontal cortex. Both systems have been implicated in SUDs and CP.
Finally, emotion is a key feature of an individual’s personal experience of CP and/or substance use. Key brain areas involved in emotional processing of stimuli include the insular and cingulate cortex. The insular and cingulate cortex are located within the lateral sulcus (i.e., Sylvian fissure) [73] and are key areas of the brain for the process of stimuli, including external stimuli and internal body sensations that might be perceived as painful [74]. These areas are also critical in forming an individual’s emotional and subjective perception of such stimuli [73]. The insular cortex has also been implicated in SUDs; specifically, during abstinence in AUD, and with an individual’s cues and responses to alcohol during abstinence [75]. Animal models further suggest shared neurobiological mechanisms underlying CP and SUD comorbidities. For example, the anterior cingulate cortex has been implicated in both socially and alcohol-mediated pain sensitivity (i.e., hyperalgesia). Lastly, tobacco use has been associated with reduced anterior cingulate and insular cortex in patients with comorbid psychiatric disorders [76], further highlighting how comorbid SUD and CP conditions might interact to challenge treatment.
Neurotransmitters and ReceptorsDifferential dopamine signaling and overall dysregulation of the reward and stress systems has been implicated for both CP and AUD (see Figure 2). There are two types of dopamine receptor release patterns, phasic and tonic. Phasic dopamine release is characterized by quick bursts of dopamine release and primarily activates D1 dopamine receptors, while tonic dopamine release is characterized by a continuous dopamine release and mostly involves D2 dopamine receptors. Chronic alcohol exposure leads to decreased phasic dopamine release and increased tonic levels of dopamine, a process hypothesized to underlie tolerance observed among individuals with AUD, such that one needs to consume more alcohol to experience the same effects [77]. The phasic-tonic dopaminergic system is similarly altered in the chronification of pain via a reward deficit state [77]. There are also several overlapping mechanisms in the stress system, including corticotropin-releasing factor receptor 1, whose activation in the hypothalamus and amygdala is associated with stress and anxiety during alcohol withdrawal (leading to increased alcohol consumption to alleviate these symptoms) [78]. Corticotropin-releasing factor receptor 1 is also associated with increased nociception and the chronification of pain [77].
NeuropeptidesNeuropeptides are chemical messengers released by neurons [79]. Within the brain, neuropeptides can modulate the activity of neurotransmitters; peripherally, neuropeptides can act as hormones and regulate various bodily functions, thereby acting over larger distances than neurotransmitters [80]. A promising neuropeptide to target for CP and SUD comorbidity is orexin. Orexin is a neuropeptide exclusively produced in the hypothalamus [81]. Studies have found that orexin may be a useful tool to reduce inflammation in CP [82], modulate pain transmission, and treat diabetes-mediated pain sensitivity (i.e., hyperalgesia) [83]. For SUDs, orexin has been associated with reward-seeking behavior in animal studies of food, morphine, and cocaine [84]. For example, one study found that orexin gene expression was downregulated in rats who were exhibiting behaviors consistent with acute alcohol withdrawal [85]. Interestingly, in models of OUD, blocking orexin-1 receptors decreased oxycodone self-administration in rats [86]. Moreover, in 2018, the National Institute on Drug Abuse (NIDA) named orexin antagonists as one of the top ten mechanisms with “highest probability of a path to FDA approval for the treatment of some aspect of OUD in the near term” [87]. In short, recent studies have highlighted orexin as a promising target for CP-SUD comorbidity.
Future DirectionsCollectively, studies demonstrate that CP and SUDs share significant neurobiological mechanisms. Together, these shared genetic and neurobiological vulnerabilities lead to increased risk for both conditions. Therefore, understanding how shared neurobiological pathways underlying CP and SUD is important to understand the etiology of their co-occurrence and to develop better treatments for their comorbidity.
Furthermore, multiple studies have linked the immune system to substance use behaviors [88,89] and CP [90]. CP lacks major biomarkers, making diagnosis and treatment development difficult. Raffaeli et al. [91] recently showcased promising results that suggest the Mu opioid receptor on B lymphocytes can be used as a biomarker for CP. Similarly, ongoing phase III clinical trials [92] show that vaccine treatments for SUDs such as cocaine use disorder and TUD might be on the horizon. Immunotherapies for SUDs would be beneficial given their specificity and prolonged effects, providing potentially lifesaving treatment for conditions (e.g., cocaine use disorder) that have no currently approved treatment. Therefore, understanding how the immune system is related to CP and SUDs can further characterization of both CP and SUDs and provide rich avenues of research to develop novel treatments for these challenging conditions.
Individuals with lower socioeconomic status (SES) are 1.32 times more likely to experience CP [93]; moreover, their pain is more severe on average than individuals with higher SES [94]. These individuals are also more likely to suffer from a SUD [95]. These associations are likely due to a combination of factors, including occupational hazards and working conditions, limited access to high quality healthcare, comorbid mental health conditions, and increased financial stress [94,96,97]. Lower SES is often accompanied by limited access to healthcare or poor-quality care, which is in turn associated with more severe pain and longer pain duration [3,94]. CP is already challenging to treat, with one study estimating the recovery rate at only 5.4% [98], and limited access to quality healthcare makes treatment even more difficult. Lower SES is also a risk factor for homelessness, which in turn increases the risk of CP and SUDs. One study in the UK found that almost two thirds of individuals experiencing homelessness suffered from CP [99]; similarly, another study estimated two out of every three people experiencing homelessness has a lifetime history of an SUD [100]. A study from Vogel and colleagues suggests that this comorbidity leads to worse treatment outcomes, finding that homeless individuals who reported daily substance use were less likely to receive professional treatment and prescribed medication for their chronic pain [99].
Neighborhood Risk FactorsNeighborhood-level factors also contribute to the risk of developing CP and SUDs [101], however, to our knowledge, no study has examined neighborhood-level risk factors leading to co-occurring CP and SUDs conditions. For example, community violence exposure has been associated with the development of problematic cannabis use in adolescents [102]; similarly, neighborhood instability (a score that aggregates multiple factors such as the percentage of vacant/rental households) increases risk for cannabis use disorder [103]. Violence in a community increases stress, leads to reduced use of urban spaces and can increase feelings of social isolation, and may worsen experiences of chronic pain [104]. Studies have found that people living in areas of greater material deprivation, air pollution, and reduced greenspace are at greater risk for CP [96,105,106] and reduced success in SUD prevention and remission efforts [107–109].
Social Support and Its Protective EffectsSocial support can be conceptualized as the emotional social assistance and comfort an individual receives from others [110]. Social support has been found to be an important protective factor against the stress response to pain [111,112]. Similarly, increased social support increases the life satisfaction [113] and probability of recovery [114,115] for individuals suffering from a SUD. While research investigating the effect of social support on individuals with co-occurring CP and SUD is more limited, a recent study by Benville et al. [116] showed that social support improves both non-cancer CP and opioid use disorder outcomes. However, authors note that individuals suffering from both CP and opioid use disorder on buprenorphine reported significantly less social support, highlighting important intricacies in the comorbidity of CP and SUDs and patients that might be at higher risk for medical complications if current support systems are insufficient. Further underscoring the importance of social support in the comorbidity of CP and SUD, it has been previously reported that individuals suffering from CP are at higher risk of experiencing loneliness, which is linked to increased risk of substance abuse. Therefore, social support is an important factor mediating the relationship between CP and SUDs.
Other Individual Risk FactorsDemographic characteristics associated with variation in incidence of CP or SUD include sex, race, and gender. For example, females are more likely to report CP than males (25.4% of females compared to 23.2% of males) [3]. Conversely, males have higher diagnosis rates of a SUD [117], while females are typically underdiagnosed with a SUD [118]. In addition, members of underrepresented racial groups, including American Indian/Alaska Native and multiracial individuals, have the highest rates of past year SUDs compared to other racial groups [119]. Like SUDs, CP is more prevalent among American Indian/Alaska Native individuals, followed by White non-Hispanic and then multi-racial non-Hispanic individuals [3]. While most opioid overdose deaths are among White individuals, the rate of drug overdoses is concerningly increasing among Hispanic and Asian groups [120]. While data is limited, research suggests that gender and sexual minority individuals have higher SUD and CP rates than heterosexual or cisgender individuals [121].
Future DirectionsGiven the greater prevalence of CP [122–124] and recent changes in substance use patterns in women, including significant increases in alcohol drinking since the COVID-19 pandemic [125,126], research is needed into the mechanisms of comorbidity and consequences for treatment and recovery specifically for women. For example, a recent study [127] revealed phenotypic and genetic associations between a female-specific pain condition, endometriosis, and depression, anxiety, and eating disorders, but no such studies have focused on female-specific pain and SUDs, to our knowledge.
Chronic pain (CP) and substance use disorders (SUDs) are serious health conditions that frequently co-occur. In this review, we have synthetized three major sources of comorbidity, namely, genetic, neurobiological, and environmental. Recent studies report evidence for a shared genetic etiology between these conditions, with moderate genetic correlations between CP and SUDs (ranging from rg = 0.2 (between multi-site chronic pain and OUD [45]) to rg = 0.63 (chronic neck/shoulder pain and OUD [46])). Other studies have shown that CP shares genetic risk with generalized addiction liability [48], as well as sharing genetic risk with specific substances such as cannabis [50]; future studies should leverage methods such as genomic structural equation modeling [128] to further parse genetic risk for CP that is shared across SUDs from genetic risk for CP that is substance-specific. Lastly, MR analyses reveal that CP and SUDs have bidirectional, causal relationships (although there is stronger evidence for CP causing SUDs) [45], further highlighting the complex interplay between these conditions. Therefore, while most studies have focused on how CP might lead to SUDs, the alternative also occurs and needs to be further investigated. Exciting new avenues of research for the genetic underpinnings of CP and SUD are on the horizon thanks to large-scale biobanks with whole genome sequencing data [53,129]. First, it is now possible to conduct well-powered rare variant studies of disease. Rare variant studies will be critical to identify large effect loci important to individual risk prediction and treatment of CP and SUDs. Finally, gene-gene and gene-environment interaction studies will further elucidate how individual genetic factors work together across the whole genomic and environmental landscape to influence individual risk, trajectory, and treatment of CP and SUDs.
CP and SUDs also have multiple overlapping neurobiological mechanisms. First, CP and SUDs are both associated with the stress [49,65] and reward brain systems [63], including the insular and cingulate cortex, prefrontal cortex, and ventral tegmental area, all areas involved in reward and stimuli processing systems. Dopamine and corticotropin-releasing factor receptor 1 are key molecules implicated in both CP [19,130] and SUDs [131,132] through their roles in the reward and stress systems. While genome-wide studies have not yet implicated specific biological pathways shared by CP and SUD, future studies with larger samples may elucidate the extent to which shared genetic liability is enriched in specific cell types, tissues, and pathways, bridging genetic evidence with neurobiological hypotheses. Two additional key areas of further research involve orexin, a neurotransmitter produced in the hypothalamus but with far-ranging modes of action across peripheral body systems. Animal and human studies of orexin have yielded promising results for its ability to manage singular CP [82,83,133] or SUD [85,134,135] diagnoses. Additional research is needed to determine how orexin can be targeted to treat concurrent CP and SUD diagnoses. Like orexin, therapies that have broad targets might be beneficial for both CP and SUD. In fact, immune therapies for individual CP and SUD diagnoses are emerging. Future studies should investigate immune-mediated treatments for individuals suffering from both CP and SUDs.
Lastly, environmental factors underlying CP-SUD comorbidity were reviewed. Socioenvironmental factors that contribute to their co-occurrence include socioeconomic background [93,95] and healthcare access [136], as well as neighborhood-level factors such as neighborhood vacancy rates [101] (although further research is needed to fully understand the influence of neighborhood-level factors on the comorbidity of CP and SUDs, rather than considering risk for each condition separately). Risk of CP and SUDs also vary by individual demographic characteristics, including race, gender and sex [137,138]. Social support is a robust protective factor for both CP and SUDs; moreover, social support is essential for successful treatment of both CP and SUDs. Given the importance of sociobiological factors such as sex in CP and SUD incidence, treatment, and recovery, future research should also focus on understudied CP conditions such as female-specific CP (e.g., endometriosis) and its relationship with SUDs. In short, there are multiple sources of genetic, neurological, and psychosocial comorbidity for CP and SUDs.
In summary, chronic pain (CP) and substance use disorders (SUDs) are debilitating and heterogeneous conditions that are difficult to treat and often co-occur. CP and SUDs share multiple sources of biological and environmental risk factors. Genetic studies point to significant shared genetic influences on CP and SUDs, highlighting the role of genetics in their co-occurrence. Genetically informed causal inference studies also highlight the fact that CP and SUDs can have bidirectional relationships. CP and SUDs share multiple neurobiological pathways, including the insular and cingulate cortex, prefrontal cortex, and ventral tegmental area, all areas involved in reward and stimuli processing systems. Socioenvironmental factors that contribute to the co-occurrence of CP and SUDs include socioeconomic background, race, gender, and neighborhood composition such as neighborhood vacancy rates. Social support is a robust protective factor against both CP and SUDs; moreover, social support is essential for successful treatment of both CP and SUDs. In short, CP-SUD comorbidity is complex; novel precision medicine models that account for the wide variety of socioenvironmental and biological factors underlying their co-occurrence will improve their prevention, diagnosis, and treatment.
Not applicable.
Conceptualization, ECJ; Methodology, PNRV and ECJ; Software, N/A; Validation, N/A; Formal Analysis, N/A; Investigation, PNRV and ECJ; Resources, ECJ; Data Curation, PNRV; Writing—Original Draft Preparation, PNRV and ECJ; Writing—Review & Editing, PNRV and ECJ; Visualization, PNRV and ECJ; Supervision, ECJ; Project Administration, PNRV; Funding Acquisition, ECJ.
ECJ and PNRV were supported by funding from NIDA (R03DA059747). ECJ also acknowledges support from K01DA051759. PNRV was also supported by the Department of Anesthesiology Training Grant R90NR021799. This manuscript is the result of funding in whole or in part by the National Institutes of Health (NIH). It is subject to the NIH Public Access Policy. Through acceptance of this federal funding, NIH has been given a right to make this manuscript publicly available in PubMed Central upon the Official Date of Publication, as defined by NIH.
No data were generated from the study.
Figure 1 was created in BioRender. Romero, P. (2025) https://BioRender.com/zmkebue.
Figure 2 was created in BioRender. Johnson, E. (2025) https://BioRender.com/yd7srma.
The authors declare that there is no conflicts of interest.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
87.
88.
89.
90.
91.
92.
93.
94.
95.
96.
97.
98.
99.
100.
101.
102.
103.
104.
105.
106.
107.
108.
109.
110.
111.
112.
113.
114.
115.
116.
117.
118.
119.
120.
121.
122.
123.
124.
125.
126.
127.
128.
129.
130.
131.
132.
133.
134.
135.
136.
137.
138.
Romero Villela PN, Johnson EC. Chronic Pain and Substance Use Disorders: A Brief Narrative Review of Genetic, Neurobiological, and Environmental Contributions to Comorbidity. J Psychiatry Brain Sci. 2025;10(3):e250003. https://doi.org/10.20900/jpbs.20250003.

Copyright © Hapres Co., Ltd. Privacy Policy | Terms and Conditions